Packaging for Double-Blinded Clinical Studies
Background
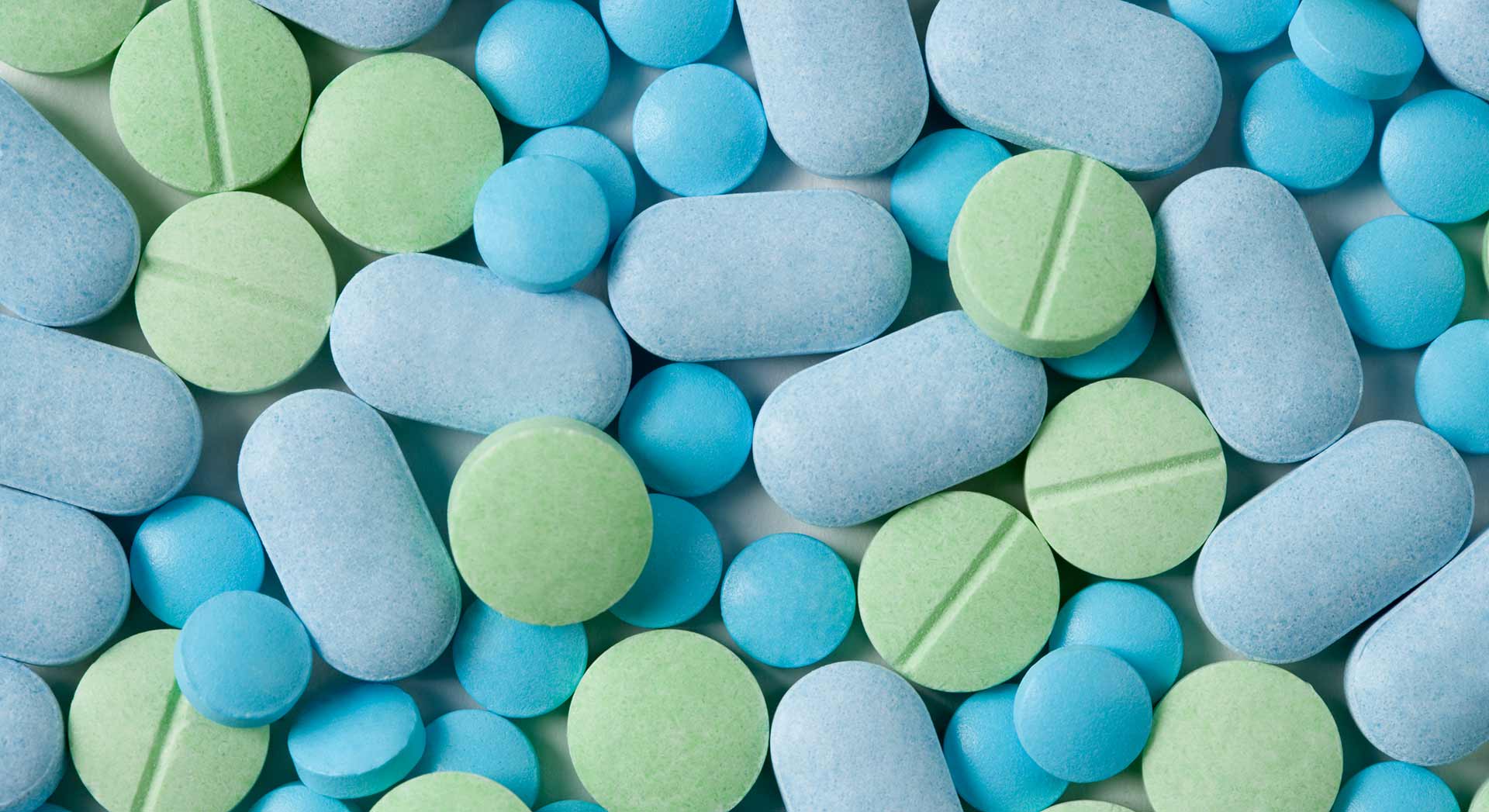
An independent, nonprofit contract research organization (CRO) required packaged product kits consisting of a variety of drug products and dosing strengths in a weekly regimen, both morning and evening dosing that changed daily, for oncology patients enrolling in double-blinded clinical studies. PGC, through its contract services, produced the clinical trial kits needed for this CRO.
Challenge
Due to the complex dosing regimens involved with four different drug components as well as matching placebos, this CRO was looking to outsource the assembly of the weekly dosing kits per specifications dictated by clinical study protocols. PGC coordinated with the major pharmaceutical manufacturer of two of the branded drug products in order for them to supply both the active drug product and the matching placebos. Double-blinded labeling was also required for the packaged kits. Owing to the number of components, PGC's quality assurance was crucial in ensuring that the precise drug product and dosage strength were correctly placed in the appropriate chamber for a particular time and day. Ensuring the integrity of the finished packaged kits was essential.
Results
PGC worked with the CRO to develop a work plan to supply initial study quantities and additional kits, as needed. The devised logistics for placing the specific drug products (active or placebo) in the appropriate compartments proved to be an advantage in completing the project quickly. Quality assurance was integrally involved with the assembly process by overseeing the ongoing process and ensuring that the clinical packaging and labeling for the finished kits were correct as designed, accurately labeled, and sealed appropriately.